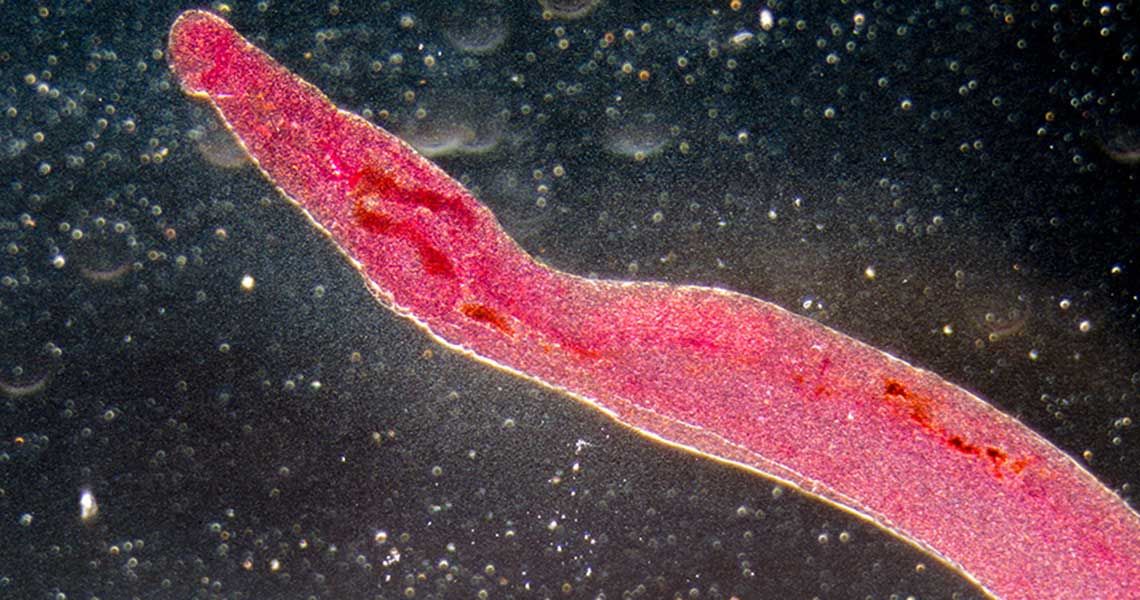
Schistosomes, a type of parasitic flatworm, infect about 200 million people worldwide every year, according to the Centers for Disease Control (CDC). Researchers at the George Washington University School of Medicine and Health Sciences (SMHS) are investigating how to manipulate the genes of these parasites to disrupt their ability to survive and infect people. In the process, they just might decipher some key details about the virology of HIV.
Researchers for some time have been trying to manipulate the genes of schistosomes, says Paul Brindley, Ph.D., professor of microbiology, immunology, and tropical medicine at SMHS. Identifying genes essential to the function and survival of the schistosomes, and targeting or disrupting them with interventions such as drugs, vaccines or even gene drives, could prevent the ability of these pathogens to cause disease, he says.
Schistosomes, also known as blood flukes, can be found in more than 75 countries in Africa, South America, the Middle East, southern China, and elsewhere. Prevalence is highest in impoverished areas where sanitary conditions are poor.
“Because it’s a tropical disease, it’s also a disease where hygiene is sub-optimal,” Brindley notes. In areas where there is a lack of sanitation infrastructure, he adds, human waste enters the environment and ultimately makes its way into the natural watercourses, which promotes disease transmission.
People become infected by schistosomes, which cause the disease schistosomiasis, when their skin comes into contact with contaminated water, contact which is frequently unavoidable in day to day activities such as bathing, laundering clothes, and children playing at the water’s edge. Infected patients at first suffer from rashes and itchy skin, according to the CDC, and then come down with fever, chills, cough, and muscle aches about a month or so after infection. If untreated, and depending on the schistosome species responsible for the infection, patients experience abdominal pain, enlarged liver and abdomen, urogenital and renal disease including passing blood in the urine, and are at increased risk for potentially life threating complications including esophageal bleeding and bladder cancer.
As a consequence, schistosomiasis is considered one of the gravest worm infections in terms of morbidity and mortality. And while there are drugs to treat schistosomiasis, Brindley says, people easily become reinfected if they go back into contaminated water.
To better understand the genetic makeup and the function of the genome of schistosomes, Brindley and his colleagues decided to attempt to infect the schistosome with HIV-1, a virus that can enter into a host’s chromosomes. But they hypothesized that HIV-1 might not have the right genetic makeup and proteins to infect a flatworm in the same fashion as it infects human cells, so the next step was to manipulate the virus to see if they could cause infection to occur.
In the lab, the researchers grow the schistosomes, which Brindley calls “complex” because of the parasite’s two-step life cycle involving freshwater snails and a mammal host. Schistosome eggs are released with a mammal’s feces or urine into water, the parasites infect snails as intermediate hosts where they multiply, and then the flatworms are released by the snails back into the water where they can penetrate a person’s skin.
Getting HIV to "Stick"
To begin exploring the schistosomes’ interactions with HIV, the researchers needed to get the virus to “stick” to the flatworm.
“When HIV infects a person, when it comes in, it’s kind of grenade-shaped with projections on the outside that allow it to attach to receptors on the right kind of human cell. It couldn’t infect, for instance, a mouse cell. It needs a specific type of human cell,” Brindley explains.
So the researchers used a well-known virological approach, called pseudotyping, to help the virus to attach to the schistosome.
“The HIV envelope itself limits the cell type that can be infected. … [B]asically HIV doesn’t infect all of the cells in the human body. So for genetic engineering studies, you might want to modify the virus to enable it to infect other types of a cell. And to achieve that, usually what is done is that you … cover the HIV particle with the envelope of another virus, which actually can enter almost any cell in the body,” explains collaborator Michael Bukrinsky, M.D., Ph.D., professor of microbiology, immunology, and tropical medicine at SMHS.
Investigators often chose the vesicular stomatitis virus to envelope the HIV cells, says Bukrinsky.
This particular protein used has a very broad host range; it’ll stick to most any cell, Brindley speculates. But “no one had really tested whether it would attach to a schistosome … but we thought we’d take a look, and it could,” he says.
Surprising Results
After the success of getting the virus to attach to the schistosome, which Brindley says was not unexpected with the pseudotyped HIV-1 virus, came the surprise: the virus on its own was able to take the next step in the process and infect the flatworm.
“I always suspected the development of the virus couldn’t happen in a schistosome,” Brindley says. “For the outside parts, we could make it look like something that would drive down the street, but I didn’t think it would actually drive far. But that has happened, and it’s fascinating for me that the virus’ biochemistry could work inside this organism.”
The reason that is so surprising is because of the very different biology of a schistosome compared to a higher organism like a human, Bukrinsky says.
For humans, the process of infection goes like this: The HIV virus, once inside a human cell, has an enzyme that allows it to convert the RNA genome to a DNA genome; otherwise known as reverse transcriptase. The converted DNA then makes its way to the nucleus and enters the chromosome thanks to the help of certain proteins. Once that occurs, the virus is transmitted by cell division and will be long-lived inside the human host.
However, schistosomes don’t have the same proteins as humans; proteins that in humans are necessary for the virus to enter into the chromosome, Brindley points out.
“Either it was, in an evolutionary sense, distantly related but close enough that it worked, or the virus was able to find whatever it could and go ‘well, I don’t have the right stuff here, but I’m going to grab this,’ ” he says.
The researchers now are testing a couple hypotheses to figure out the mechanics of the integration.
It’s possible the schistosome does have similar enough proteins to those that support HIV replication and integration in higher organisms, says Bukrinsky, or maybe HIV replication in schistosomes occurs through a completely different process than that of humans.
In addition to exploring how HIV integrates and replicates inside a schistosome, the researchers are looking at whether the process happens in nature, without laboratory manipulation — and what that may mean for people who are coinfected with both HIV and schistosomiasis.
That’s something the researchers are eager to find out.