-
Hookworm
-
The GW VRU is part of the Texas Children's Hospital Center for Vaccine Development (TCH-CVD), a Product Development Partnership (PDP) that is developing and testing novel, low-cost vaccines to help prevent disease caused by hookworm infection. Hookworm infection causes anemia and can lead to problems with physical and cognitive development in children.
We recently completed a clinical trial of the Na-GST-1 hookworm vaccine candidate at GW (see Clinical Trials). In this trial, we immunized healthy volunteers with our candidate vaccine, then gave them a dose of hookworm larvae in a controlled human infection model (CHIM) to test for the protective effect of the vaccine.
Background
Hookworms are parasitic nematodes (roundworms) that are transmitted via contact with contaminated soil. Soil-transmitted helminths infections are amongst the most prevalent human infections worldwide and afflict primarily the poorest and most disadvantaged communities. The hookworm species that most commonly infects humans is Necator americanus, whereas Ancylostoma duodenale and Ancylostoma ceylanicum are more focally clustered in certain regions.
Hookworm infection is one of the most common chronic infections of humans and affects more than 700 million people in tropical countries, in particular in Southeast Asia, China, sub-Saharan Africa and the Americas.
The areas most afflicted are impoverished rural areas, where approximately 3.2 billion people worldwide are at risk of hookworm infection.
Hookworm Transmission
Hookworm infection is spread via eggs that are shed in the feces of an infected host and contaminate soil in areas with poor sanitation. Within one or two days, hookworm larvae hatch from the eggs. The first larval stage, called L1 or rhabditoform larvae, is not infective and feeds on the microbial content of the soil. After two molds, the larvae develop into the infectious L3 or filariform larvae, which stop feeding on bacteria and have much increased their mobility.
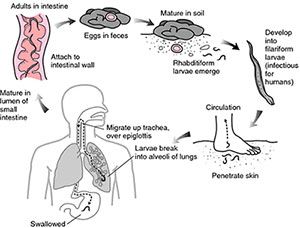
Upon contact with human skin, the infectious larvae penetrate the skin and travel via the blood stream to the lung, where they are coughed up and swallowed. Eventually the larvae settle in the small intestine, where they mature to male and female adult hookworms and attach themselves to the intestinal wall to feed on blood.
Adult hookworms can live in the human intestine for many years. Heavy hookworm infections cause severe intestinal blood loss that results in chronic iron-deficiency anemia and malnutrition, which is particularly devastating for pregnant women and children. Hookworm infection of pregnant women can result in severe anemia, increased maternal morbidity and prematurity or even loss of the fetus, whereas children with a medium or heavy hookworm burden suffer from growth retardation, and can develop intellectual, cognitive and educational deficits. As they grow into adulthood, many children with chronic hookworm infections experience a substantial decrease income-earning potential. As a result, countries with a high prevalence of hookworm infections suffer from reduced productivity and poor socioeconomic development.
Development of a Human Hookworm Vaccine
The general approach to hookworm control worldwide has been the frequent and repeated administration of benzimidazole anti-hookworm drugs to infected individuals in high-prevalence areas.
However, this approach is limited by high re-infection rates and the development of drug-resistant hookworm populations. These concerns have prompted interest in alternative and more permanent tools for hookworm control, such as the development of a vaccine. Developing a vaccine against a relatively large, multicellular organism is however particularly challenging.
The adult hookworm living in the human intestine is dependent on the digestion of hemoglobin from erythrocytes (red blood cells) for use as an energy source. The current strategy for the development of a vaccine against human hookworm infection is focused on parasitic enzymes required for blood feeding, hemoglobin digestion and heme detoxification.
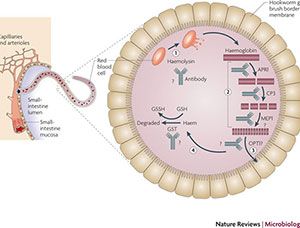
Hookworms digest hemoglobin via a proteolytic cascade that begins with the enzyme aspartic protease 1 (APR-1), which cleaves the intact hemoglobin and allows for further hydrolysis of the fragments by other proteases. Vaccination with recombinant APR-1 has protected animals from infection in challenge studies.
Another antigen from the hookworm blood digestion pathway that is a promising vaccine target is glutathione S-transferase-1 (GST-1), an enzyme that binds and detoxifies the toxic oxygen byproducts of the hemolysis. Hamsters immunized with recombinant GST-1 of N. americanus (Na-GST-1) and later challenged with infectious larvae were significantly protected compared to unimmunized animals.
Future Studies
The aim is to induce antibodies against these two enzymes that are essential for the hookworm blood feeding pathway, thereby reducing both host blood loss and the number of hookworms attached to the gut. Both vaccine candidates are currently in phase 1 clinical trials designed to evaluate their safety and immunogenicity. The goal is to license a hookworm vaccine that is comprised of both antigens, Na-APR-1 and Na-GST-1, by 2020. If both recombinant vaccine candidates are shown to be safe and immunogenic in the current phase 1 trials in adults and children, they will be combined to one product and tested in phase 2 and 3 trials in hookworm endemic areas in Brazil and Sub-Saharan Africa to evaluate their efficacy in preventing hookworm infections.
-
COVID-19
-
The GW VRU is a participating site in several clinical trials investigating the safety, efficacy, and immunogenicity of COVID-19 vaccines, including the groundbreaking Phase 3 trial of the Moderna mRNA-1273 COVID-19 vaccine that was FDA-authorized in 2021 and used in an unprecedented vaccination effort throughout the US and worldwide to combat the COVID-19 pandemic. GW enrolled 349 adults between August and October 2020, in collaboration with the team of Dr. Manya Magnus of the GW Milken Institute of Public Health, and as part of the CoVPN network. FDA granted Emergency Use Authorization for this vaccine in December 2020.
The GW VRU enrolled 109 adults in Sanofi Pasteur's Phase 2/3 study of a SARS-CoV-2 Recombinant Protein Vaccine with AS03 Adjuvant in 2021-2022. GW VRU is currently working with the Division of Microbiology and Infectious Diseases (DMID) on a Phase 2 COVID-19 Adaptive Immunologic Landscape Trial (COVAIL) to study prototype and variant vaccine candidates against new variants as they emerge.
- Monkeypox
-
The GW VRU is participating as a site for a Phase 2 dose-sparing evaluation study (DoSES) for the Jynneos monkeypox vaccine (see NIH press release). The study will evaluate two intradermal (ID) regimens for Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN) vaccine compared to the standard subcutaneous (SC) regimen in healthy, vaccinia-naïve adults 18 to 50 years of age.
-
HIV Vaccine Development
-
The GW VRU is partnering with the International AIDS Vaccine Initiative (IAVI) to conduct early-phase clinical trials of experimental HIV vaccines.
-
Schistosomiasis Vaccine Development
-
The GW VRU is also collaborating with the TCH-CVD to develop a novel vaccine to prevent intestinal and hepatic schistosomiasis caused by Schistosoma mansoni. Currently, the GW VRU is conducting a Phase 1 trial of the Sm-TSP-2/Alhydrogel vaccine in healthy adults in collaboration with its partners, the Fundação Oswaldo Cruz (FIOCRUZ) in an endemic area of the Brazilian state of Minas Gerais. This study is being funded by the US National Institute of Allergy and Infectious Diseases (NIAID) through its contract the the Baylor College of Medicine Vaccine and Treatment Evaluation Unit (VTEU).
- Zika
-
The GW VRU partnered with LEIDOS Biomedical and the National Institutes of Health (NIH) on a Phase II trial of a DNA-based Zika vaccine (VRC-ZKADNA090-00-VP), at sites spread across countries where people have been infected by the virus (protocol VRC-705). The trial at one of those sites, in Belo Horizonte, Brazil, is being run by faculty from the GW School of Medicine and Health Sciences in collaboration with Brazilian investigators at the Hospital das Clínicas and FIOCRUZ. The two main objectives of the study are to test the safety of the vaccine in the population and to induce an immune response that would prevent an infection with the virus.
The Zika virus is primarily transmitted to humans through the bite of infected Aedes aegypti mosquitoes and can be transmitted from an infected pregnant woman to her baby during pregnancy, which can result in serious birth defects, including microcephaly. The importance of a DNA-based vaccine is that it can be used in pregnant women who cannot receive live viral vaccines. The vaccine was created by scientists at the National Institute of Allergy and Infectious Diseases.
The goal in Belo Horizonte was to enroll at least 100 participants aged 15 to 35 years in the trial; actual enrollment has been 138. Each participant received a series of three injections over the space of four months and was subsequently followed for safety and efficacy. The volunteers are required to participate in frequent specimen collections of urine and blood to test for the virus. The other study sites are located in the United States, Peru, Ecuador, Puerto Rico, Costa Rica, Panama, Columbia and Mexico.