Jump to: Ongoing Lab Projects
Anti-viral antibodies
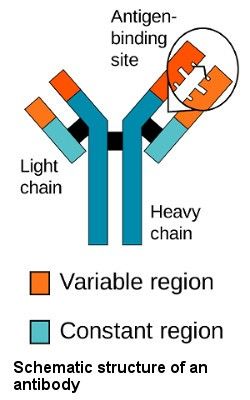
A vaccine is generally made by inducing the human immune system to produce antibodies against individual fragments or “antigens” of an invading pathogen that will then neutralize the pathogen and aide in its destruction by immune cells. Antibodies have a variable region designed to bind to all different kinds of antigens (we focus on antibodies targeting viral proteins). They also have constant regions designed to interact with immune cells and “flag” pathogens or infected cells for destruction.
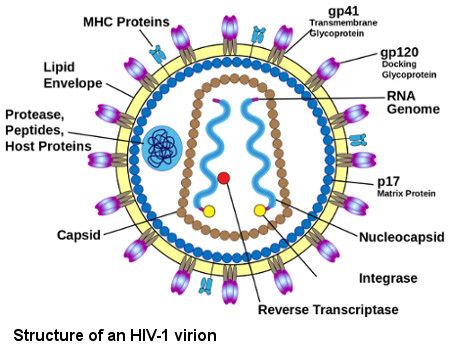
Human Immunodeficiency Virus type 1 (HIV-1) is one of the most devastating diseases in the world. Today, 38 million people globally live with HIV, a prevalence of 0.8% of the world population (https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics). There is no readily available cure for HIV infection; however, a new generation of drugs and treatments can enable people living with HIV today to have longer and healthier lives. The administration of combination antiretroviral therapy (ART) suppresses viral replication and slows disease progression, but it cannot eradicate HIV because the virus is ’hidden’ in latently infected cells, which contain the integrated viral genome without producing viral protein to alert the immune system. In the absence of ARTs, the virus reemerges from these hidden reservoirs within a few weeks.
Ongoing Lab Projects
The Lynch lab aims to investigate the impact of broadly neutralizing antibodies on both HIV virions and HIV-infected cells to explore new avenues for the development of potent preventative and therapeutic agents against HIV infection. Our lab also studies antiviral antibody responses in emerging infectious diseases, such as Zika Virus and SARS-CoV-2.
1. What role could broadly neutralizing antibodies play in cure strategies? The isolation of broadly neutralizing antibodies against HIV-1 has renewed interest in antibody treatment for chronic infection. Viral escape during monoclonal antibody treatment, however, remains a concern. One class of broadly neutralizing antibodies in particular, the VRC01-class, targets the highly conserved CD4 receptor-binding site (CD4bs) that is critical for viral function and can induce antibody escape mutations that negatively impact the ability of HIV-1 to replicate (Lynch et al., J Virol., 2015). We have recently demonstrated in human trials that VRC01 can induce escape mutations in the virus of chronically infected individuals (Lynch et al., Science Transl Med., 2015). As human trials with combinations of multiple bNAbs are in progress, questions related to virus escape must be answered for the design of optimal monoclonal antibody therapy. We are currently mapping virus escape from bNAbs using in vitro replication assays to assess selection pressure of various bNAb combinations on genetically diverse viruses, including non-subtype B viruses.
2.What is the role of HIV-1 diversity in sensitivity to treatment with monoclonal antibodies? ART can successfully suppress HIV-1 replication, however, patients on ART must maintain consistent therapy in order to avoid rebound from the latent viral reservoir. Strategies are urgently needed to clear or limit this reservoir. Although individuals infected for longer periods prior to ART appear to harbor greater genetic diversity in their viral reservoir, more studies are needed to explore the role viral diversity plays in the potential efficacy of treatment with broadly neutralizing antibodies. To do so, we have characterized HIV-1 genetic diversity in individuals with varying lengths of infection, as well as the sensitivity of their inducible virus reservoir to broadly neutralizing antibodies (bNAbs).
3.Is the autoimmune diagnosis of GBS during Zika infection associated with a different antibody response? We recently published (Lynch et al., JID., 2019) that people who developed Guillain-Barré Syndrome (GBS) after Zika infection had higher neutralizing antibody titers one-month after infection compared to people who did not have GBS. These results suggest an increased inflammatory environment or increased antigen exposure. We are currently studying if the antibodies are not just quantitatively different but also qualitatively.
4.What is the role of antibodies to SARS-CoV-2 in long COVID? The Lynch Lab is collaborating with the GWU COVID-19 Specimen Bank and Long COVID clinic to accelerate research on the transmission and pathogenesis of COVID-19. The Lynch Lab processes and analyzes samples to assess subjects’ antibody responses to COVID-19 infection according to clinical severity. Additionally, the Lynch Lab is studying antibody responses to COVID-19 vaccines in immunocompromised populations.