Jump to: Toxoplasmosis | Microsporidiosis | Current lab projects
Toxoplasmosis
Toxoplasmosis is a disease caused by the intracellular protozoan parasite Toxoplasma gondii, one of the most common parasites infecting humans worldwide. Estimates suggest that up to one-third of the global population is chronically infected with Toxoplasma gondii. In the United States, approximately 22% of the population 12 years and over is afflicted with toxoplasma, whereas up to 95% of populations in other regions of the world have been shown to be infected.
In the United States, toxoplasmosis is considered to be the 2nd leading cause of death due to foodborne illness, and was therefore included in a group of five Neglected Parasitic Infections targeted by the Center for Disease Control and Prevention (CDC) as priorities for public health action.
Toxoplasma gondii is a parasite that infects most warm-blooded animals, including humans. In healthy persons, toxoplasma infection usually causes either no symptoms or only mild flu-like symptoms due to the ability of the immune system to keep the parasite from causing disease. However, in pregnant women and immune-compromised individuals such as AIDS patients, the infection can lead to serious conditions. In these patients, toxoplasma infection can cause neurological diseases and encephalitis and can affect the liver, heart, ears, and eyes.
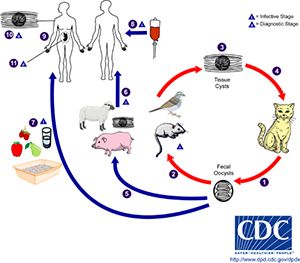
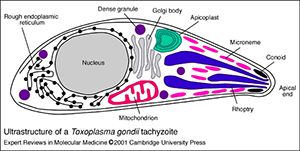
The only known primary hosts for the parasite are cats, but it can asexually reproduce in any warm-blooded animal. Cats play an important role in spreading toxoplasmosis through shedding of infectious oocysts in their feces. Intermediate hosts, including birds, rodents, and animals bred for human consumption, become infected by contact with soil, water or plant material contaminated with toxoplasma oocysts in their environment. Shortly after ingestion, the oocysts develop into the mobile, quickly multiplying tachyzoites that are responsible for the main parasitic expansion in the host. Following the initial rapid phase of proliferation, tachyozoites convert into the slowly dividing bradyzoites that reside in the neural or muscle tissues of the host and form tissue cysts. Cats can become re-infected by consuming birds or mice infected with tissue cysts.
Transmission of Toxoplasma gondii happens via different routes. The most important are consumption of contaminated undercooked meat or vegetables, direct transmission from animals to humans (zoonotic), frequently via contact with contaminated feces of domestic cats, or in utero from mother to fetus (congenital transmission). In rare occasions, toxoplasma can be transmitted via blood transfusion or organ transplantation. Transmission from mother to child through the placenta during pregnancy can have serious consequences for the unborn child that range from diseases of the nervous system and eyes to abortion of the fetus or birth of a stillborn child.
Microsporidiosis
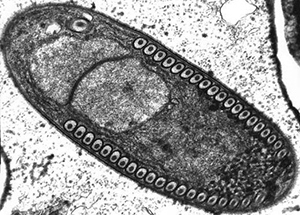
Microsporidia is a term used to describe a large group of more than 1200 highly divergent intracellular unicellular fungi that parasitize a wide range of vertebrate and invertebrate hosts. For a long time, microsporidia were classified as protozoa and have only recently been attributed to the kingdom of fungi. Microsporidia have the smallest known eukaryotic genomes.
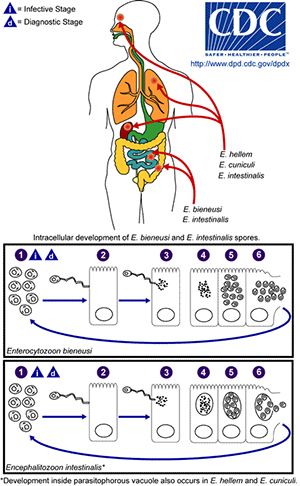
In animals, microsporidia frequently cause debilitating diseases rather than lethal infections. Parasitic microsporidia have long been known to have a large impact on commercially exploited insects, fish, and farm animals. Approximately 10% of all microsporidia are parasites of vertebrates, including a minimum of 15 species that have been recognized as human pathogens. The resulting disease microsporidiosis is an intestinal infection that causes diarrhea and can result in severe wasting in immune-compromised individuals such as AIDS patients. Before the AIDS pandemic, microsporidia were rarely identified as the cause of human disease, but recently it has become apparent that microsporidiosis is also a problem in certain groups of non-HIV-infected people including children, the elderly, travellers, organ transplant recipients, cancer patients, and diabetics.
Transmission of microsporidia is believed to occur mainly via the fecal-oral route, with infected humans and animals or contaminated food and water to be the main source of infection. The main routes of transmission are inhalation, ingestion, skin trauma, congenital and sexual transmission. Microsporidia are characterized by the production of highly resistant spores that vary in size dependent on the species. Mature spores from species infecting humans are very small and range from 0.8μm to 5μm, a size similar to many bacteria. The spore contains a coiled polar filament or tube that unfolds and injects the sporoplasmic contents of the spore into the host cell under appropriate conditions of changes in pH or osmotic pressure. After the infective sporoplasm was transferred to the cell it undergoes two developmental stages, and the host cell eventually raptures and releases new infective spores into the environment.
Current lab projects
Over the years the focus of the Khan laboratory has been to study the development and maintenance of CD8+ T cell immunity during Toxoplasma gondii infection. The role of CD8+ T cell subsets in controlling chronic infection against this parasite is well established. Our laboratory has described the role of γ-chain cytokines, especially IL-15, in the induction and persistence of robust memory CD8+ T cell responses (Khan et al., J Exp Med, 2002).
Chronic toxoplasmosis is a major problem for HIV-infected and other immune-compromised individuals. The latest studies from our laboratory have demonstrated that during chronic toxoplasmosis CD8+ T cells, especially the memory population, exhibit increased expression of inhibitory markers like PD-1 and lose their functionality, resulting in the reactivation of latent infection. Administration of anti-PDL-1 antibody restores the CD8+ T cell response and animals are able to maintain the infection in a latent stage (Bhadra et al., PNAS, 2011, Bhadra et al., J Immunol, 2011).
Recently, we have made two important observations: 1) Once anti-PDL-1 treatment is stopped, CD8+ T cells’ dysfunctionality reappears and animals exhibit mortality due to toxoplasmic encephalitis. 2) Dysfunctional CD8+ T cells express other inhibitory markers in addition to PD-1, in particular LAG-3, 2B-4 and CD160. Interestingly, combinatorial blockade of PD-1 and LAG-3 results in prolonged survival even after the treatment has been stopped. The current focus of the laboratory is to investigate the antibody cocktail that can be administered for minimal time and can efficiently and irreversibly restore CD8+ T cell function. In addition, supplemental therapies, such as agonist CD40 administration that can be used along with anti-PDL-1 therapy, are also being studied.
The laboratory is also interested in exploring the priming of CD8+ T cells at the start of an infection and evaluating the strategies that result in total parasite clearance so that chronic infection is not established.
As reported several years ago by our laboratory, CD4+ T cells are not primary effector cells during infection, although they play an important secondary role in the maintenance of the CD8+ T cell response, as reported several years ago by our laboratory (Casciotti et al., Infect Immun., 2002). We have recently observed that chronic infection compromises the functionality of CD4+ T cells, which could be an important cause for CD8+ T cell exhaustion and dysfunctionality. Although the helper role of CD4+ T cells in establishing CD8+ T cell immunity is well known, the mechanism remains somewhat elusive and could be dependent on the infection. This is an area of active research.
Our laboratory has also been investigating the protective mechanisms during infection with Encephalitozoon cuniculi, a microsporidia species relevant for human disease. Being an oral pathogen, E. cuniculi induces a strong intraepithelial lymphocyte (IEL) response in the gut mucosa.
Our group reported that CD8αβ IELs in the gut compartment are of critical importance for clearing the infection in that tissue (Moretto et al., J Immunol., 2004). The mechanism involved in the priming of the CD8αβ IEL response during infection is actively being investigated in our laboratory.
In our studies, we have observed an important role for IL-21, a cytokine produced by different T cell subsets, in the development of peripheral CD8+ T cell effectors against E. cuniculi infection. The precise role of IL-21 in the development of CD8+ T cell immunity in response to infection is currently being evaluated.
Furthermore, using an aging mouse model, we have recently reported that in an aging mouse model an increase of the down-regulatory cytokine TGFβ1 compromises the induction of the polyfunctional CD8+ T cell effector response (Bhadra et al., J Clin Invest, 2014). The mechanism involved in the TGFβ-mediated attrition of CD8+ T cell effector responses is being studied.