Many parasites, including schistosomes, have evolved to produce molecules which manipulate host biology in order to promote propagation of the parasites’ life cycle. An excellent example of one such molecule is the Interleukin-4-inducing Principle from Schistosoma mansoni Eggs (IPSE, Schramm et al.), originally named alpha-1 (McLaren et al.), and later also known as the S. mansoni chemokine-binding protein (smCKBP), as termed by Smith et al.
View 3D Crystal structure of IPSE/alpha-1 from Schistosoma mansoni eggs
IPSE is only IPSE is only expressed by schistosome eggs (Schramm et al.), is secreted into the environment (Mathieson and Wilson and Smith et al.), and has multiple host modulatory properties. First, as its name suggests, IPSE within soluble egg antigen can induce IL-4 release from basophils of schistosomiasis-naïve humans (Falcone et al.) and mice in an IgE-dependent fashion (Schramm et al.). In vitro work indicates IPSE activates NF-AT in IgE-bearing basophils, which is consistent with IPSE’s ability to trigger IL-4 release from these cells:
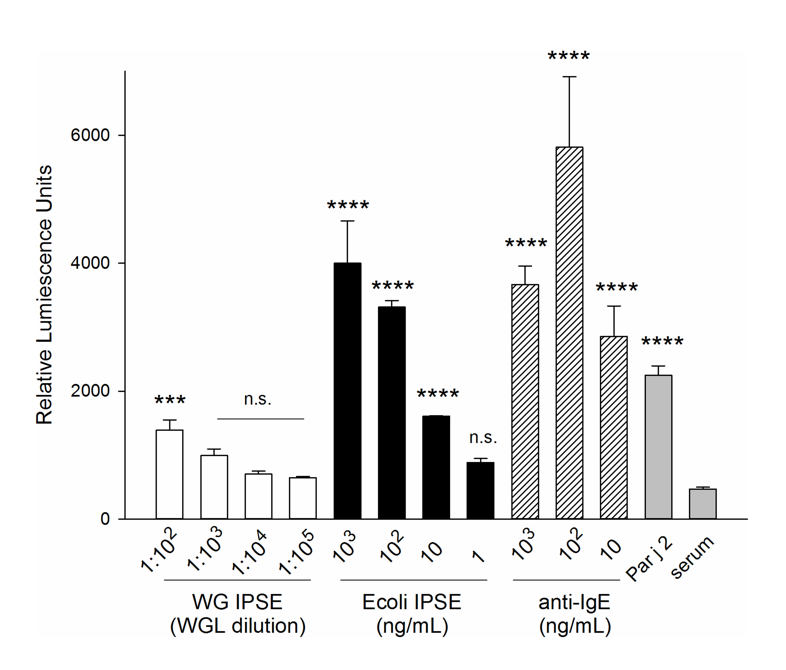
All cells (except IgE only) were sensitized for 16 hours with pooled serum from P. Judaica-allergic individuals diluted 1∶50 (v/v). The experiment included positive controls: sensitized with 1 µg/mL IgE and stimulated with the indicated amounts of polyclonal anti-human IgE (hatched bars); stimulated with Par j 2 (100 pg/ml; grey bar), as well as the negative controls (grey bas): serum sensitized/unstimulated cells (serum only). Data are mean ±SD of the readings of three separate wells. Multiplicity adjusted p-values were obtained by ANOVA followed by Dunnett’s test for each condition compared with serum only control ****: p<0.0001; ***: p<0.001; n.s.: not significant.
Other work demonstrated that IPSE was a pepsin-sensitive, heat stable, 30-40 kDa glycoprotein (Wuhrer et al., Meevissen et al., and Meevissen et al.) that binds to IgE (Haisch et al. and Xie et al.), possibly through a crystallin fold (Meyer et al. and Meyer et al.).
IPSE has also been reported to potentially induce IL-10 secretion by Bregs (B regulatory cells) by binding antibodies on the surface of these cells (Haeberlein et al.).
Another host modulatory property of IPSE is its capacity to bind to and neutralize the activity of multiple chemokines (Smith et al.). It is possible that this activity of IPSE allows it to “mold” schistosome egg granuloma progression, since induction or administration of anti-IPSE antibodies leads to increased granuloma size in the mouse liver (Fahel et al.) and lung (Smith et al.).
Perhaps the most astonishing ability of IPSE is its ability to enter host cell nuclei through the action of a nuclear localization sequence (Kaur et al.). It is possible that once in host cell nuclei, IPSE induces changes in gene transcription.
The importance of IPSE in schistosomiasis-associated immune responses was underscored by Oliveira et al., who reported that individuals living in schistosomiasis-endemic areas and yet resistant to infection had higher titers of anti-IPSE IgE antibodies.
IPSE’s profound immunomodulatory properties may have implications that extend beyond schistosomiasis. Li et al. observed that pig fibroblasts engineered to produce IPSE and injected into mice shifted the xenogeneic immune response to a type 2 immunity-associated profile. These workers speculate that IPSE could be used to prevent rejection of xenogeneic transplants. Doenhoff et al. and Igetei et al. demonstrated that antibodies to peanut allergens cross-react with antibodies to IPSE, which they conjecture is consistent with the hygiene hypothesis (the theory that allergies are becoming more common in the developed world because our immune systems are no longer “primed” by parasites and other infectious agents present in less clean environments):
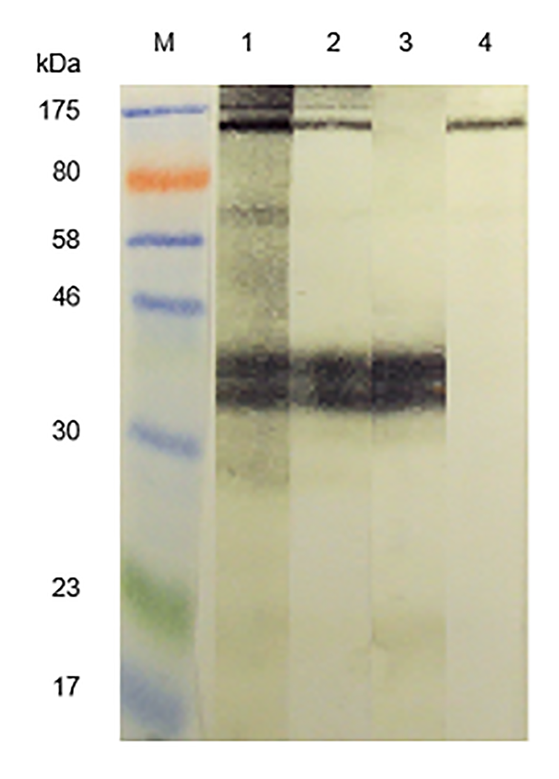
From Doenhoff et al. Western blot immunoreactivity of acid-eluted antibodies and control rabbit antisera monospecific for S. mansoni egg antigens; IPSE/alpha-1 and kappa-5 against S. mansoni egg antigens. M = molecular size markers. Primary antibodies: 1 = anti-SmSEA; 2 = anti-SmSEA antibodies eluted from the 43 kDa latex molecule; 3 = rabbit antiserum specific for S. mansoni egg antigen IPSE/alpha-1; 4 = rabbit antiserum specific for S. mansoni egg antigen kappa-5
We have cloned the Schistosoma haematobium orthologs of IPSE (hereafter referred to as H-IPSE, Pennington et al.):
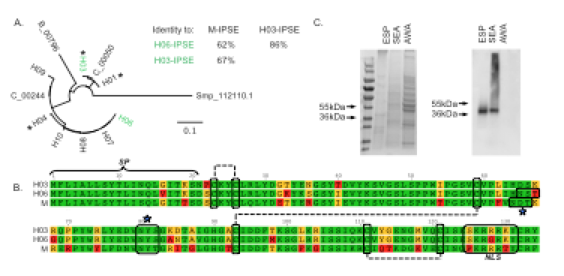
Although there are multiple H-IPSE orthologs predicted to be present in the S. haematobium genome, they cluster into two main clades exemplified by H03-IPSE and H06-IPSE (panel A). These orthologs exhibit >60% identity to M-IPSE (S. mansoni ortholog of IPSE) and 86% to each other, and feature a signal peptide, multiple disulfide bonds, and a nuclear localization sequence like M-IPSE (panels A and B). H-IPSE orthologs are only found in egg-secreted protein and soluble egg antigen (panel C), and mRNA is only expressed in eggs and adult female worms (which carry eggs):
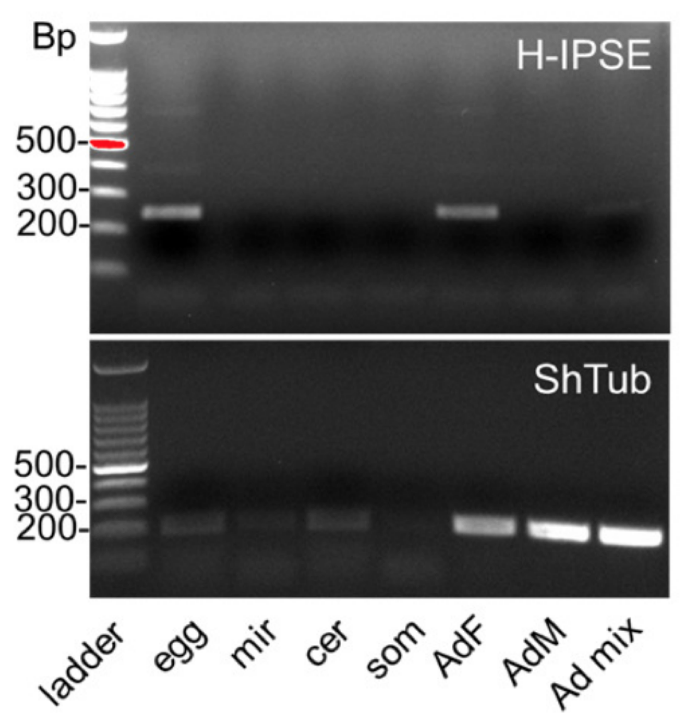
Stage -specific expression of H-IPSE mRNA. RT-PCR results for H-IPSE obtained from cDNAs, prepared by reverse transcription of DNAse-treated RNA of various life stages of S. haematobium. Ladder: 100 basepair (Bp) DNA ladder; egg: S. haematobium egg cDNA; mir: miracidial cDNA; cer: cercarial cDNA; som: in vitro mechanically transformed schistosomula cDNA; Ad, F, M mixed cDNA from female, male or mixed adult worms, respectively. ShTub: control housekeeping gene, S. haematobium tubulin.
We expressed M-IPSE and H-IPSE in mammalian HEK293-6A cells, which allows for glycosylation of the recombinant proteins:
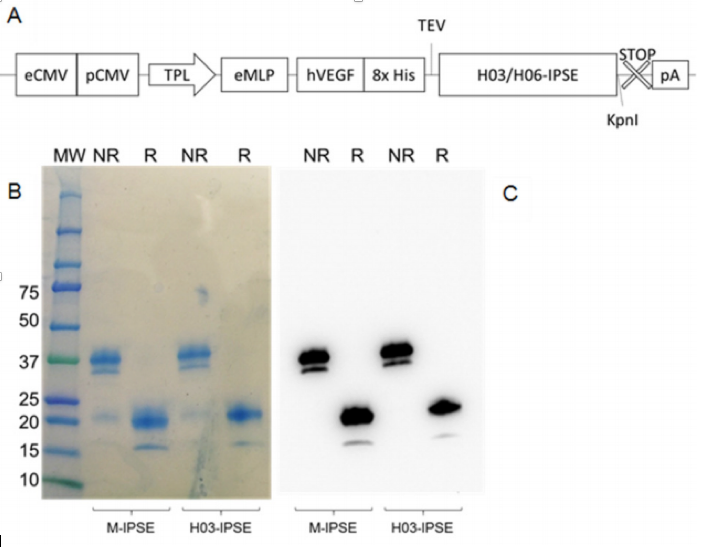
Expression of M-IPSE and H-IPSE in HEK293-6A cells. A) Schematic diagram of pTT5 H03/06 -IPSE expression cassette. eCMV=Cytomegalovirus (CMV) 801 enhancer sequence; pCMV=CMV promoter; TPL=tripartite leader sequence from adenovirus; eMLP=enhancer element from the adenovirus major late promoter (MLP); hVEGF=human vascular endothelial growth factor signal sequence; 8xHis=octahistidine tag; TEV=Tobacco Etch Virus protease cleavage site; STOP: stop codon; pA: β -globin poly adenylation signal. B) Coomassie -stained 4 -20% SDS – PAGE gradient gel and C) Western Blotting of recombinant H03 -IPSE (and M -IPSE, used as comparison) expressed in HEK 293SF -3F6 cells and purified by IMAC from serum -free culture supernatant, and run under non -reducing (NR) or reducing (R) conditions.
We then incubated H03-IPSE and H06-IPSE with HTB-9 urothelial cells and confirmed that these proteins localized to host cell nuclei:
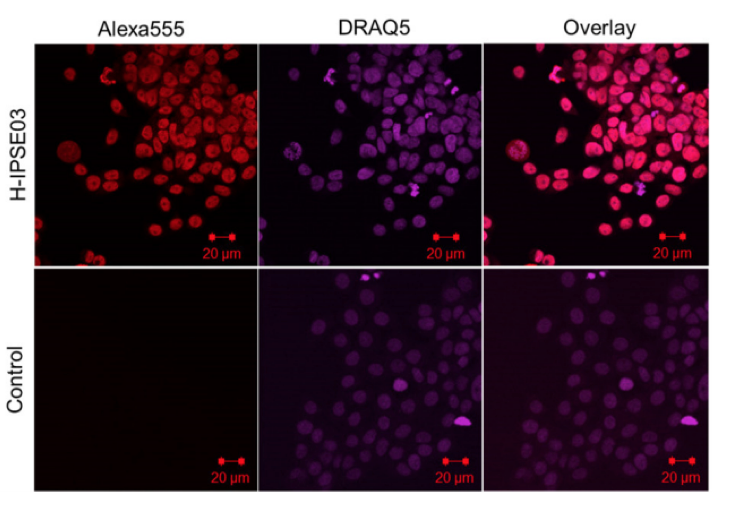
Recombinant H03 -IPSE is taken up by HTB -9 host cells and translocates to the nucleus. HTB -9 cells, incubated for 24 hours with 0.40 nM recombinant H03 – IPSE, were stained with 5 μM DRAQ5 nuclear stain for 15 minutes at room temperature, followed by staining with a mouse anti -His antibody and Alexa Fluor® 555 conjugated Goat anti -Mouse IgG (H+L) as secondary antibody. The right column shows the overlay of the two channels. The uptake in HTB -9 cells was visualized by confocal microscopy. The primary anti -His antibody was omitted in the control lane.
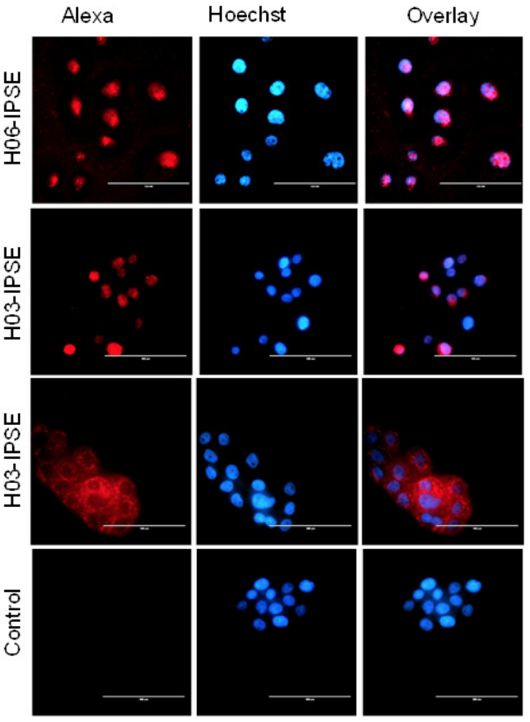
Fluorescence microscopy of HTB -9 cells incubated with recombinant H03 – IPSE (NLS: SKRRRKY), H06 -IPSE (NLS: SKRGRKY) or H03 -IPSE mutant (NLS: 820 SKAAAKY). HTB -9 cells were stained with Hoechst 33342 nuclear stain for 15 minutes at room temperature, followed by staining with a mouse anti -His antibody and Alexa Fluor® 555 -conjugated Goat anti-Mouse IgG (H+L) as secondary antibody. The right column shows the overlay of the two channels. The primary anti – His antibody was omitted in the control lane. Bar size is 100 μm.
The nuclear localization sequences of H-IPSE were even able to direct a large, tetrameric GFP construct to host cell nuclei, but this was dependent on an intact sequence:
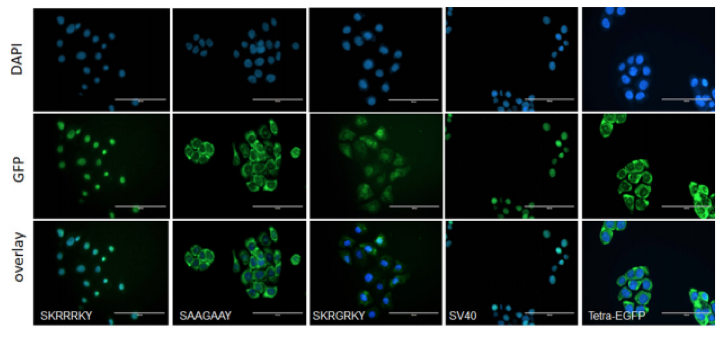
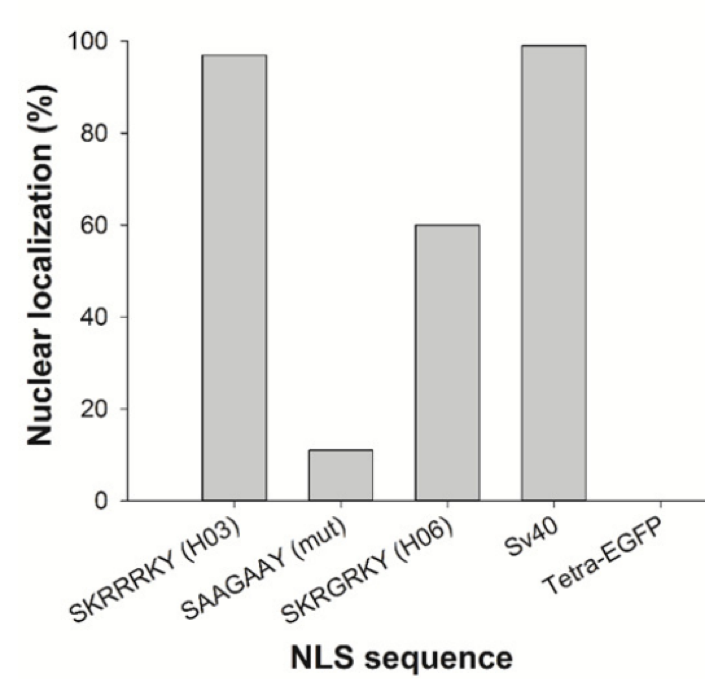
Effect of multiple amino acid substitutions on NLS in H -IPSE. A) The nucleotides encoding the H0 3/H0 6 -IPSE nuclear localization sequence (SKRRRKY and SKRGRKY, respectively) were inserted into the pTetra -EGFP construct (2,3). pTetra -EGFP encodes a tetrameric EGFP construct resulting in the expression of a fluorescent protein which due to its size ( >100 kDa) is excluded from the nucleus in the absence of a functional NLS (Tetra -EGFP) or imported into the nucleus in the presence of a functional NLS (canonical SV40 NLS, H0 3/H0 6 -IPSE NLS). Nuclei were stained with DAPI and green fluorescence measured with the GFP light cube on an EVOS fl microscope , 24 hours after transfection. Bar is 100 μm. B) Comparison of wild -type H06 -IPSE, H03 -IPSE and H03 -IPSE mutant NLS effect on nuclear localisation of Tetra -EGFP fusion protein. One hundred transfected HTB9 cells were evaluated under the EVOS fl microscope for each transfection and the percentage of cells displaying exclusive nuclear fluorescence, as opposed to cytosolic only or mixed cytosolic/nuclear localization, recorded. Positive control: Sv40 canonical NLS sequence; negative control: unmodified Tetra -EGFP vector (Tetra -EGFP).
In summary, the S. haematobium genome possesses multiple IPSE orthologs, which are expressed in vivo and feature nuclear localization properties akin to M-IPSE. Given the potent immunomodulatory properties of M-IPSE, we have demonstrated the ability of H-IPSE proteins to favorably modulate inflammation and pain in multiple bladder disease models.
IPSE as a Therapeutic
We have gone on to show that H06 H-IPSE (one of the Schistosoma haematobium orthologs of IPSE) is a potential anti-inflammatory drug and analgesic. In our first paper we demonstrated that a single dose of IPSE inhibits urothelial damage, inflammation, and hemorrhage caused by ifosfamide, a chemotherapeutic drug.
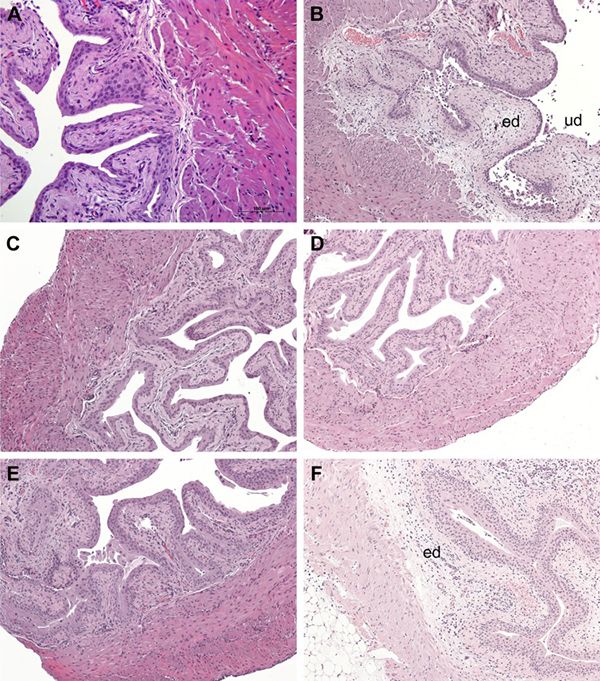
H-IPSE mitigates ifosfamide-induced histopathological changes in the bladder. Histologic assessment of bladders from mice receiving the following: saline only (A), ifosfamide only (B), MESNA and ifosfamide (C), recombinant IL-4 and ifosfamide (D), H-IPSE and ifosfamide (E), and H-IPSE + neutralizing anti-IL-4 antibody and ifosfamide (F). Bladders from the saline-treated group showed normal urothelium with no sign of edema (ed) or urothelial denudation (ud). Ifosfamide-injected bladders exhibited segmental urothelial denudation, severe edema with effacement of the submucosa and occasional frank hemorrhage. Ifosfamide-injured bladders exposed to H-IPSE, IL-4, or MESNA showed intact urothelium and overall preserved bladder architecture with a relative lack of edema. The protective effect of H-IPSE was reversed by prior administration of neutralizing anti-IL-4 antibody.
In a subsequent publication we showed that H03 H-IPSE, another S. haematobium ortholog of IPSE, also inhibits ifosfamide-induced hemorrhagic cystitis. Finally, we have used RNA-Seq in this model to demonstrate that IPSE likely exerts this therapeutic effect by inhibiting gene pathways related to IL-1, IL-6 and TNF:
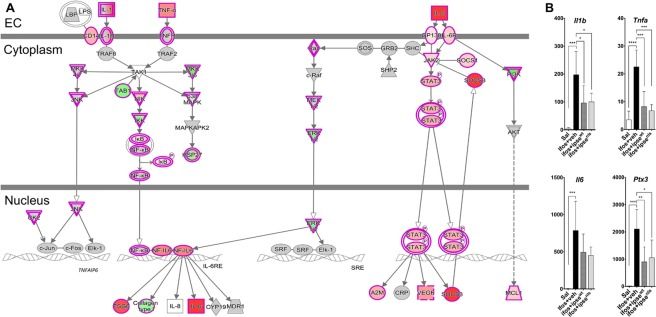
The IL-1β, TNFα, and IL-6 pathways, a major inflammatory pathway upregulated in the bladder during ifosfamide-induced hemorrhagic cystitis was downregulated by IPSE pretreatment. (A) Pathway and functional analyses were generated through the use of IPA57. Bladders of ifosfamide-exposed mice upregulated expression of genes from the IL-1β, TNFα and IL-6 pathways and their corresponding receptors and downstream nuclear transcriptional factors. In the case of IL-1β and TNFα, these cascades converge upon NFκB. IL-6 also indirectly interacts with NF𝜅B through ERK1/2 activation of NF-IL6, which works with NFκB to promote transcription of target genes. Keys: upregulation (red), downregulation (green), cytokines (square), growth factors (dotted square), phosphatase (triangle), kinases (inverted triangle), transmembrane receptors (ellipse), transcriptional regulators (wide circle), peptidase (rhombus), group or complex (double lined shapes), transporter (trapezium), acts on (line with filled arrow), translocate (line with open arrow), inhibition (line with perpendicular line at edge). (B) Il1b, Tnfa, Il6 and Ptx3 gene transcription were upregulated by 2–3 orders of magnitude in the bladders of ifosfamide-treated mice. Pretreatment with IPSE or its NLS mutant reduced the levels by ~50% relative to the ifosfamide-treated group. Similar trends were observed for cognate receptors and downstream transcription factors (data not shown).
We have also observed that IPSE alleviates bladder pain caused by resiniferatoxin, the most potent agonist for the capsaicin pain receptor:
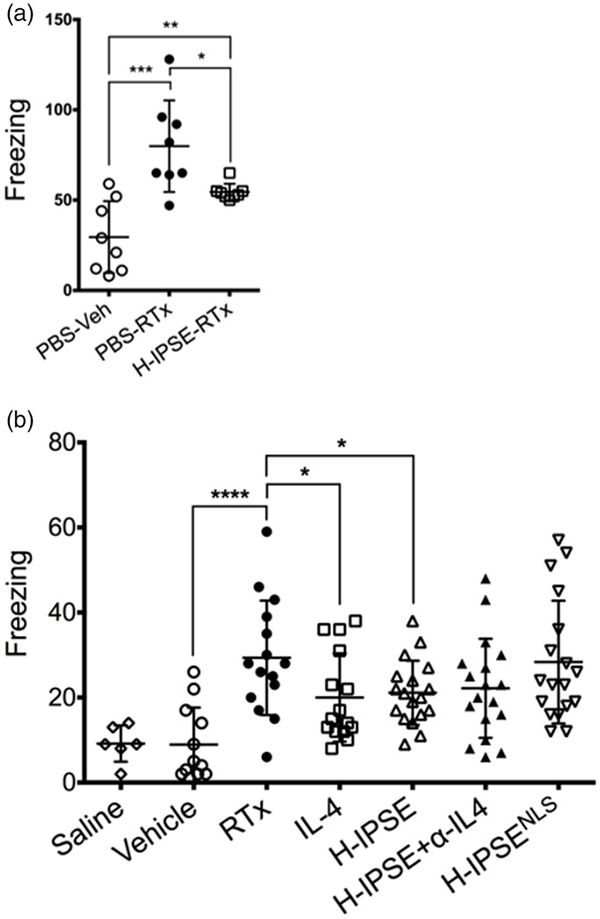
IPSE reduces resiniferatoxin-induced, pain-related freezing behaviors in an IL-4- and nuclear localization sequence-dependent manner. (a) Mice were administered intravenous phosphate-buffered saline (PBS) followed by intravesical PBS/Tween/ethanol vehicle (“PBS-Veh”), intravenous PBS with intravesical resiniferatoxin in vehicle (“PBS-RTx”), or one intravenous dose of the H06 H-IPSE ortholog of IPSE 24 hours before intravesical resiniferatoxin in vehicle (“H-IPSE-RTx”). (b) Mice were given intravenous PBS and intravesical PBS (“saline”), intravenous PBS and intravesical PBS/Tween/ethanol vehicle (“Vehicle”), intravenous PBS and intravesical resiniferatoxin in vehicle (“RTx”), recombinant IL-4 given intraperitoneally followed by intravesical resiniferatoxin in vehicle (“IL-4”), the H06 H-IPSE ortholog of IPSE given intravenously 24 hours before intravesical PBS with resiniferatoxin in vehicle (“H-IPSE”), the H06 H-IPSE ortholog of IPSE 24 hours given intravenously and anti-IL-4 antibody given intraperitoneally 30 minutes before resiniferatoxin in vehicle administered intravesically (“H-IPSE+α-IL4”), or a nuclear localization sequence (NLS) mutant of H06 H-IPSE given intravenously 24 hours before resiniferatoxin in vehicle administered intravesically (“H-IPSENLS”).
Lastly, we have noted that IPSE decreases bladder inflammation triggered by urinary tract infections:
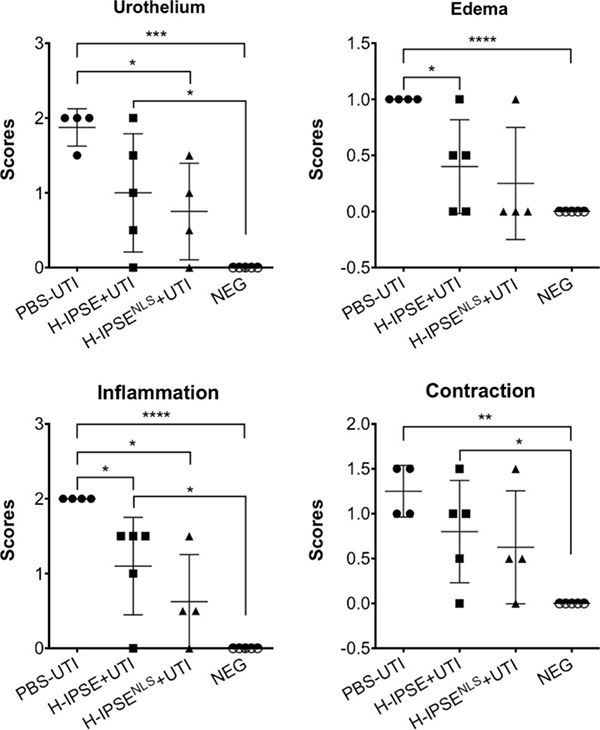
Effects of H-IPSE on UTI-associated bladder pathogenesis. H-IPSE reduces bladder pathogenesis. “PBS-UTI”: mice given PBS vehicle tail vein injections prior to UTI challenge; “H-IPSE+UTI”: mice administered the H06 ortholog of IPSE before UTI challenge; “H-IPSENLS-+UTI”: mice given a nuclear localization sequence mutant of the H06 ortholog of IPSE prior to UTI challenge; “NEG”: unmanipulated mice. ANOVAs with Tukey’s multiple comparisons test were used for comparisons between group means with every other group. Bars represent means and standard deviation. Findings were considered statistically significant at p < 0.05. *, **, ***, and **** denote p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively. Data are representative of two independent experiments.
We are continuing to develop IPSE as a therapeutic for other immune-mediated diseases.