Obtaining cercaria-snails start shedding some time between day 40 and 50 when snails are kept at ~24°C
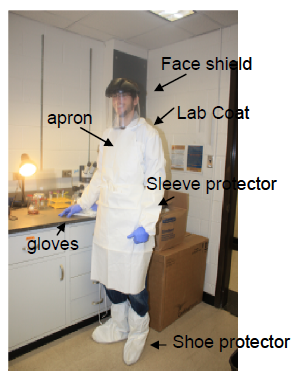
1. Wear protective attire-lab coat+disposable water resistant apron, sleeve protectors, shoe covers, gloves. During this process, a full face shield must be worn. In addition, a spray bottle containing 70% ethanol must be readily at hand.
2. Before cercarial shedding begins the snails are placed in a dark incubator for 24-48 hours.
3. Transfer the snails with forceps to a new tank of fresh clean dechlorinated water to rinse the snails off. This helps remove any food, feces and some rotifers.

4. With a pair of forceps, remove the infected snails from the aquaria into a large #20 sieve (keep the snails in a monolayer). Avoid transferring debris with the snail.

5. Using a garden sprayer (Flo-Master pressure sprayer purchased at Home depot) filled with DI water, spray the snails to remove rotifers over a sink. The first time snails are shed will require extensive washing to remove rotifer contamination. After they have been shed several times less effort is required. (rotifers effect the quantity and quality of the cercaria that are .

6. Place the snails in a clean beaker with dechlorinated water. 17-20 snails/ 30-40 ml -It is better to have a bit more water keeping the snails completely covered. Too little water will lead to snail stress and negative results. The amount of water depends on the number of snails and the size of the beaker. If multiple tanks (25 snails per tank) of snails are being shed ~150ml of water in a 500 ml beaker is used.

7. Place under a lamp with a 60W equivalent fluorescent bulb ( you do not want to heat the snails up with an incandescent lamp bulb-even with the fluorescent bulb heat is generated so be aware)
8. (This step is probably only necessary if this is the first shed for the snail group) After 2 min look at the water with a dissecting microscope-there will not be many cercaria but if rotifers are still present you will see them. If rotifers are there return the snails to the sieve and rewash with the Flo-master.
9. Expose the snails to light for 30-45 min checking occasionally to determine if cercaria are shedding. If this is the first shed for the group of snails they may not be shedding. If they are not shedding return them to the aquaria rather than stressing the snails by leaving them under the bright light.
10. Snails that are overstressed will turn the water a brown or red/brown color. If this happens you will have snail die-off and the surviving snails will produce mucous during the shed where the cercariae that are shed clump together and cannot be separated readily from the mucous.
11. After the first 30-45min the cercarial water is collected carefully avoiding snail feces and put in a clean beaker at RT and an additional 30-40 ml of shedding 4 water is added to the snails. The snails are again exposed to light for 45 min. This process is done 3-4 times total- approximately 2 hours of total cercarial shedding.
12. After 2 hours carefully with forceps remove the snails from the beaker and return them to the aquaria. Once shedding, snails are shed twice a week until they stop.
13. Remove the remaining water containing cercaria carefully trying to avoid feces (you will get some but try to be careful so that it does not break up into small bits so that it can be removed by filtering).
NB: Decant the cercarial water carefully into a second beaker before passing through the filter leaving some of the snail feces in the first beaker, this step helps eliminate additional (potential contaminants).

14. Pass the cercaria through the 47 uM stainless steel Millipore screen (cat# XX1004730) apparatus into a beaker to remove any debris/snail feces.
15. Transfer the filtered cercaria into 50 ml conical tubes. Place these tubes on ice-the cercaria pellet better after being on ice.
16. Spin the tubes in an eppendorf 5810R at 2,200 rpm (974Xg) for 15 min at 4°C.
Notes: Do not treat any apparatus with bleach or subsequent experiments will result in dead snails or cercaria. Discarded snail water can be treated with bleach.
Cercarial Transformation
all work is carried out in a biological safety cabinet from here on
- In the biological safety cabinet, remove the supernatant from the pellet of cercariae using the vacuum apparatus (this must be done immediately before the cercariae become motile again).
- Resuspend the cercariae in 5 mls 37°C 1XPBS + 2% antibiotic/antimycotic (Invitrogen cat # 15240) by shaking/tapping. Do not use a pipet to resuspend the pellet because the cercariae are sticky and will be difficult to get out of the pipet. The PBS is warm to aid in resuspension.
- Tap the 50ml tube to dislodge the pellet and resuspend the cercariae.
- Transfer cercariae into 15 ml tubes. Add more warm 1X PBS+ 2% antibiotic/antimycotic (PSF) to the empty 50 ml tube to recover any remaining cercaria.
- Spin down at 2200 rpm for 5 min at 4°C. The cold centrifuge helps to pellet the cercaria.
- Take off as much of the supernatant as possible leaving a bit on top of the pellet, and combine cercarial pellets into one tube if possible.
- Repeat this washing step 3-5 times
- Resuspend the pellet in 5-7 ml 37°C somule wash after the final wash. Invert tube to resuspend the pellet.
Somule Wash:
500ml DMEM (Invitrogen cat#11995)
5 ml 1M Hepes (Fisher cat# BP310-500)
10ml PSF (Invitrogen cat# 15240) - While performing the final spin, prepare 1 Petri dish, 2X 10ml syringes and 22uM emulsifying needle (Popper & Sons cat# 7975)
- Transfer the resuspended pellet to a 50 ml conical tube so that it can be loaded into one of the 10 ml syringes. Alternatively pour the resuspended pellet directly 6 into the 10 ml syringe. (yet another alternative is to resuspend the pellet of cercaria in 5 ml somule wash and put it in the petri dish then draw it up into the 10 ml syrine- Add an additional 2 ml somule wash and draw it up into the syringe for a total or 7 ml).
- Fit the 22G emulsifying needle into the 10 ml syringe. Connect the other end into the second 10 ml syringe.
- Passage cercariae through the emulsifying needles ~15X (15 full count, 30 pushes). Check a small drop under the microscope to see the efficiency of tail shearing.
Alternative Protocol #1
- After cercariae have been sheared, empty syringes into 15 ml tube with fresh somule wash.
- Centrifuge somules for 5 min at 1500 rpm.
- Prepare Percoll gradient in 15 ml tubes. We normally make half of the recipe below, and divide into (2) tubes (10 ml each).
Percoll Gradient:
- 24ml percoll
- 4ml 10x PBS
- 1ml HEPES
- 1ml P/S/F
- 10ml dH2O
- After the spin, remove supernatant and resuspend into 1 ml of somule wash.
- Add ~500ul of somules to each percoll gradient, being careful not to disturb the gradient.
- Centrifuge gradient for 10 min at 1500 rpm. Acceleration and brake set to 1.
- After the spin, remove the supernatant/percoll and move the pellet of somule heads to a new 15 ml tube. Resuspend in 10 mls of somule wash.
- Centrifuge somules for 5 min at 1000 rpm. Remove supernatant and repeat the wash two more times. 3X washes total.
- After last wash, resuspend the somule pellet in warmed somule medium (see below). Divide somules among wells of a 6-well plate, 4 ml media per well.
Alternative Protocol #2
- After cercariae are emulsified, empty the syringe into one well of a 6 well plate
- Swirl plate gently to allow somules to settle and tails to float.
- Remove supernatant carefully
- Add somule wash 4-5 ml.
- Repeat until most of the tails are removed.
- Remove somule wash and replace with somule media (see below)
- Split somules to more wells if needed (25,000-30,000 somules/well). For any experiment as few as 500 somules can be used up to 30K/experimental group.
The day after the tails are removed from the cercaria perform a gravity wash: (perform in biological safety cabinet)
- Transfer the somules to a 15 ml tube.
- Fill the tube with 1X 37°C 1X PBS+2%PSF-mix by inversion
- Place tube in 37°C Incubator for 30 min
- Remove supernatant by vacuum aspiration
- Repeat once time if the somules are not clean
- Finally resuspend somules in warm somule media and return to the incubator
(37°C with 5%CO2)
Washing and Feeding
- Change somule media ~every other day. Once a week, do a media change by gravity wash to change the blood.
- Media change by centrifuge:
- Transfer somules to 15ml tube and wash wells with somule wash.
- Fill tubes up to ~10mls and centrifuge for 5 min at 1000rpm.
- Remove supernatant and resuspend somules in ~4ml warmed somule media and move back into 6-well plates.
- Media change by gravity wash:
- Transfer somules to 15ml tube and wash wells with somule wash.
- Let the somules settle by gravity for ~30 mins (move them back into the 37C incubator).
- Remove supernatant and resuspend somules in ~4ml warmed somule media and move back into 6-well plates.
Red Blood Cells
- Wash the red blood cells 4X in 1 X PBS wash. Resuspend in ~2-5ml somule wash, depending on the amount of RBCs.
- Feed the somules between 1-5ul of the washed RBCs, depending on the amount of RBCs. Start feeding them ~3days in culture, remove the old blood and give them fresh blood once a week.
Flash freeze cercariae pellet
- After spinning down the cercarial water, remove the supernatant and flash freeze the pellet with liquid nitrogen or dry ice.
- Store in –80 freezer.
Cercariae are ready to work with approximately 2 days after their tails have been removed (1 day after the gravity wash-however, they can be used immediately after the gravity wash if necessary).
| 1L | 500ml | 250ml | Item | Stock (working conc) | Storage |
|---|---|---|---|---|---|
| 810.5ml | 405.25ml | 202.625ml | Basal Medium Eagle | 1X (1X) | 4 |
| 1g | 0.5g | 0.25g | Lactalbumin hydrolysate | (1g/L) | 4 |
| 1g | 0.5g | 0.25g | Glucose/Dextrose | (1g/L) | RT |
| 500ul | 250ul | 125ul | Hypoxanthine | 1mM (0.5uM) | -20 |
| 1ml | 500ul | 250ul | Serotonin | 1mM (1 uM) | -20 |
| 1ml | 500ul | 250ul | Insulin | 8mg/ml (8 ug/ml) | 4 |
| 1ml | 500ul | 250ul | Hydrocortisone | 1mM (1 uM) | -20 |
| 1ml | 500ul | 250ul | Triiodothyronine | 0.2mM (0.2 uM) |
-20 |
| 5ml | 2.5ml | 1.25ml | MEM Vitamins | 100X (1X) | 4 |
| 50ml | 25ml | 12.5ml | Schneider's medium | 1X (5%) | 4 |
| 10ml | 5ml | 2.5ml | HEPES | 1M (10mM) | 4 |
| 100ml | 50ml | 25ml | Serum | 1X (10%) | -20 |
| 20ml | 10ml | 5ml | P/S/F | (1X ) | -20 |
| Item | Company | Catalog # |
|---|---|---|
| Basal Medium Eagle | Invitrogen | 21010 |
| Lactalbumin hydrolysate | Sigma | L9010 |
| Glucose/Dextrose | Sigma (Fisher) | D9559 (BP350-1) |
| Hypoxanthine | Sigma | H9636 |
| Serotonin | Sigma | H9523 |
| Insulin | Sigma | I9278 |
| Hydrocortisone | Sigma | H0888 |
| Triiodothyronine | Sigma | T6397-100mg |
| MEM Vitamins | HyClone (Invitrogen) | SH30599.01 (11120-052) |
| Schneider's medium | Invitrogen | 11720-034 |
| HEPES | Fisher | BP310-500 |
| Serum (FBS) | HyClone | SH30088.03 |
| Antibiotic/antimycotic (PSF) | Invitrogen | 15240 |